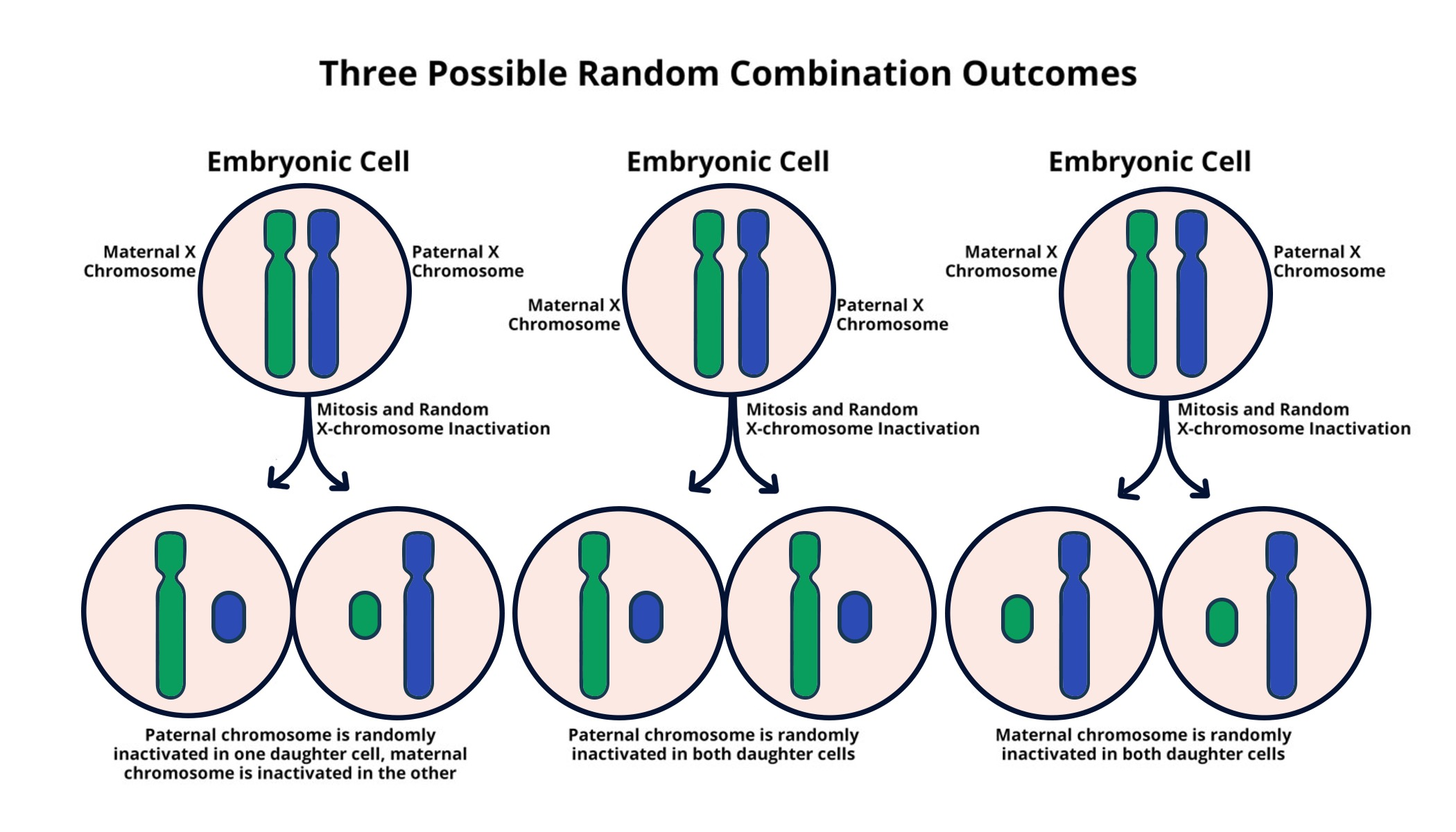
X-inactivation mechanisms play a crucial role in ensuring gene dosage balance between males and females. In females, the presence of two X chromosomes means that one must be silenced, a process that has puzzled scientists for decades. Recent research by Jeannie Lee and her team at Harvard Medical School provides insight into this complex chromosomal silencing, which has significant implications for understanding various X-linked diseases such as Fragile X Syndrome and Rett Syndrome. By investigating how the X chromosome is inactivated, researchers hope to develop new treatments that can target mutations affecting this vital chromosome. This knowledge not only sheds light on fundamental genetic processes but also opens the door to potential therapies for conditions stemming from X-linked disorders, benefiting countless individuals affected by these challenges.
Exploring the intricacies of chromosomal silencing, particularly the mechanisms of X chromosome inactivation, reveals a fascinating aspect of genetic regulation in mammals. This process, essential for achieving genetic equilibrium between genders, involves sophisticated cellular strategies that strategically turn off one of the two X chromosomes in females. The ongoing research efforts led by scientists like Jeannie Lee are instrumental in unlocking the mysteries surrounding this phenomenon, with significant implications for the treatment of X-linked diseases such as Fragile X and Rett syndromes. By understanding these alternative pathways to gene expression control, researchers are in a unique position to explore therapeutic options that could correct or alleviate the impacts of specific genetic mutations. As investigations continue, it is becoming increasingly evident that advancements in this area may usher in a new era of targeted gene therapies.
Understanding X-Inactivation Mechanisms
X-inactivation is a vital process that ensures dosage compensation between males and females by silencing one of the two X chromosomes in females. This intricate mechanism is not just a mere biological necessity; it holds significant implications for understanding and potentially treating X-linked diseases such as Fragile X Syndrome and Rett Syndrome. The studies conducted by Jeannie Lee’s lab at Harvard have illuminated the pathways and molecular interactions involved in this chromosomal silencing, unveiling how certain substances within cells dictate gene expression based on their chromosomal status.
At the core of this process lies Xist, an RNA molecule crucial for initiating the inactivation of one X chromosome. When Xist interacts with the gelatinous substance surrounding chromosomes—referred to playfully as ‘Jell-O’—it alters the material properties of this gel, making it more pliable. This flexibility is essential for the introduction of other silencing molecules that navigate into the chromosome’s structural complexities, effectively quelling its activity. The nuanced understanding of these X-inactivation mechanisms not only sheds light on a fundamental genetic principle but also provides potential therapeutic pathways for conditions arising from X-linked mutations.
The Role of Chromosomal Silencing in Genetic Disorders
Chromosomal silencing is a critical mechanism for regulating gene expression, particularly for X-linked genetic disorders. Disorders such as Fragile X Syndrome, characterized by intellectual disability, and Rett Syndrome, a neurodevelopmental disorder affecting females, are direct consequences of mutations on the X chromosome. Jeannie Lee’s research focuses on how chromosomal silencing can be influenced and potentially lifted, allowing access to the healthy alleles that may be dormant on the affected X chromosome. The possibility of revitalizing these inactivated genes opens up new avenues for treatment.
The findings from Lee’s team indicate that by manipulating the interaction of Xist and the surrounding chromatin environment, scientists may be able to reactivate genes that were previously silenced due to mutations. The promise of these approaches is substantial: not only could they pave the way for advanced therapies for Fragile X Syndrome and Rett Syndrome, they also offer hope for improved understanding of X-linked diseases. As research progresses, the insights gained could transform how we approach gene therapy and chromosomal aberrations.
Implications for Therapeutics in X-Linked Diseases
The potential therapeutic implications stemming from Jeannie Lee’s work on gene activation are immensely promising for individuals affected by X-linked diseases. By targeting the mechanisms of X-inactivation and chromosomal silencing, her lab aims to develop treatments that reactivate silenced genes responsible for conditions like Fragile X and Rett Syndromes. As these strategies evolve, they offer a tantalizing glimpse into the future of genetic medicine, where gene therapies could become a standard practice in treating complex disorders caused by genetic mutations.
As research continues, Lee emphasizes the importance of safety studies before moving to clinical trials. The mechanisms that allow for gene unsilencing must be thoroughly understood to ensure that therapeutic interventions do not inadvertently disrupt healthy gene function. The goal is to harness this newfound understanding of X-inactivation to create targeted treatments that activate dormant genes without affecting the normal functioning of other X-linked genes. This meticulous approach could minimize side effects, making gene therapy a viable option for many patients.
Jeannie Lee’s Groundbreaking Research on Chromosomal Function
Jeannie Lee’s extensive research over the past decades has significantly advanced our understanding of chromosome biology and its implications for human health. Her investigations into how X-chromosomal inactivation occurs have unraveled complex dynamics that govern gene expression across sex-based genetic differences. By elucidating the gelatinous environment surrounding chromosomes, Lee’s lab has provided critical insights that push the boundaries of genetic research and its applications to medicine.
The advances in understanding X-inactivation are not just academic; they hold transformative potential for developing therapies for X-linked diseases. Lee’s focus on practical applications demonstrates the relevance of her work, showing how scientific discovery can lead directly to potential clinical treatments. This bridges the gap between theoretical research and patient care, leading to innovations that may revolutionize how we approach genetic disorders in the future.
Advancements in the Treatment of Fragile X Syndrome
Fragile X Syndrome remains one of the more prevalent hereditary causes of intellectual disabilities. Jeannie Lee’s recent findings pave the way towards innovative treatment strategies specific to this condition. By focusing on the X-inactivation process, her lab explores how to reactivate the healthy X chromosome, potentially reversing the effects of the mutation responsible for Fragile X. What is particularly exciting is the prospect of these findings contributing to therapies that might not only alleviate symptoms but address the root cause.
The research highlights a crucial turning point in our ability to not just silence problematic genes but also to awaken the dormant, healthy copies of those genes on the inactivated X chromosome. This approach could fundamentally change the outlook for individuals with Fragile X Syndrome, offering a hope for treatment options that were previously considered unattainable. Continuous research is necessary, but the implications are promising for both immediate and long-term outcomes in genetic therapy.
Exploring the Complexities of Rett Syndrome
Rett Syndrome is a complex neurodevelopmental disorder, primarily affecting females, characterized by normal development followed by developmental regression. Jeannie Lee’s exploration of X-inactivation mechanisms offers a novel perspective on this condition. By investigating the processes behind gene silencing and the interaction between Xist and chromatin, Lee is working towards understanding how to reactivate genes that are critical for proper neuronal function and development.
Through her research, Lee is bringing attention to the potential for therapeutic strategies to awaken silenced genes associated with Rett Syndrome. These advancements not only suggest possibilities for reversing some of the symptoms associated with the disorder but also underscore the need for further investigation into the genetic underpinnings of neurodevelopmental conditions. If successful, these therapies could provide transformative benefits for patients and families grappling with the challenges of Rett Syndrome.
Gene Activation: A New Frontier in Gene Therapy
As the understanding of X-inactivation mechanisms deepens, the potential for gene activation emerges as a promising frontier in the field of gene therapy. Jeannie Lee’s research suggests that by manipulating the RNA-centric processes involved in chromosomal silencing, it might be possible to activate silenced genes associated with various X-linked diseases. The dual challenge of understanding how to activate these genes while ensuring minimal disruption to healthy gene function is at the forefront of Lee’s ongoing research efforts.
This focus on gene activation represents a paradigm shift in how we can approach the treatment of genetic disorders like Fragile X Syndrome and Rett Syndrome. Pioneering strategies that allow for targeted gene expression have the potential to transform therapeutic regimens, moving from broad treatments to more precise interventions tailored to individual genetic profiles. As such, the future of gene therapy could be on the cusp of dramatic advancements fueled by the insights gained from the study of X-inactivation.
The Intersection of Genetics and Medicine
The intersection of genetics and medicine is increasingly becoming a focal point of scientific inquiry, especially with emerging research like that of Jeannie Lee. As researchers continue to uncover the complex dynamics of X-inactivation and its implications for diseases such as Fragile X and Rett Syndrome, the potential for clinical applications becomes more tangible. Understanding the genetic basis of these conditions not only helps guide treatment strategies but also frames how we think about prevention and early intervention.
With advancements in gene therapy and chromosomal biology, there is an increasing understanding that conditions previously thought to be purely genetic in nature can be altered through targeted therapies. As such, ongoing research is poised to enhance the integration of genetic insights into everyday medical practice, creating a more nuanced approach to treating genetic disorders and leveraging the power of our genetic makeup to improve health outcomes.
Future Directions in X-Chromosome Research
The ongoing research into the mechanisms of X-inactivation promises exciting future directions for genetic science. As Jeannie Lee and her colleagues delve deeper into the intricacies of chromosomal silencing and its implications for X-linked diseases, there is potential for groundbreaking discoveries that could contribute to more effective treatments. A better understanding of how to activate silenced genes could not only improve outcomes for patients with Fragile X Syndrome and Rett Syndrome but also open doors to new therapies for other genetic disorders.
Future studies will undoubtedly focus on refining the techniques for gene activation, assessing their applicability in clinical settings, and determining the long-term effects of manipulating X-inactivation mechanisms. As research continues to progress, the collaboration between geneticists, biologists, and medical professionals will be crucial in translating these findings into real-world therapies, ultimately transforming the landscape of genetic medicine and providing hope to individuals and families affected by genetic disorders.
Frequently Asked Questions
What are the mechanisms of X-inactivation in relation to Fragile X Syndrome?
X-inactivation is a critical biological process that occurs in females, wherein one of the X chromosomes is inactivated to prevent gene dosage imbalances. In Fragile X Syndrome, an X-linked disorder caused by mutations in the FMR1 gene, understanding the X-inactivation mechanisms can provide insights into therapeutic approaches. Research from Jeannie Lee’s lab suggests that manipulating X-inactivation may help alleviate symptoms of Fragile X by unsilencing the healthy X chromosome.
How does Jeannie Lee’s research contribute to understanding X-linked diseases?
Jeannie Lee’s research focuses on the mechanisms of X-inactivation, providing foundational knowledge that could transform our understanding of X-linked diseases like Fragile X Syndrome and Rett Syndrome. By dissecting how Xist RNA modifies chromosomal silencing, her work paves the way for potential treatments that could reactivate silenced genes associated with these disorders.
What role does chromosomal silencing play in Rett Syndrome?
Chromosomal silencing through X-inactivation is particularly relevant in Rett Syndrome, an X-linked neurodevelopmental disorder. In females, the presence of one healthy and one mutated X chromosome can lead to a partial or full loss of function in genes encoded on the X chromosome. By investigating X-inactivation mechanisms, researchers like Jeannie Lee aim to develop strategies to unsilence these critical genes, potentially offering new therapeutic avenues for Rett Syndrome.
Can X-inactivation mechanisms be targeted for therapeutic interventions in X-linked diseases?
Yes, targeting X-inactivation mechanisms offers a promising strategy for therapeutic interventions in X-linked diseases. Recent work by Jeannie Lee’s lab demonstrates that compounds could potentially unsilence the inactivated X chromosome, restoring function to genes involved in disorders such as Fragile X Syndrome and Rett Syndrome. This approach could lead to innovative treatments that utilize the body’s natural processes of chromosomal silencing.
What is the significance of the Jell-O-like substance in X-inactivation mechanisms?
The gelatinous substance surrounding chromosomes plays a crucial role in X-inactivation mechanisms by providing an environment where chromosomal silencing can occur effectively. Jeannie Lee’s research highlights how this ‘Jell-O’ alters in response to Xist RNA, facilitating the inactivation of one X chromosome in females. Understanding this biophysical aspect of X-inactivation can lead to breakthroughs in therapies for X-linked diseases.
How does the understanding of X-inactivation help in developing treatments for Fragile X Syndrome?
By elucidating the mechanisms of X-inactivation, researchers have identified potential methods to reactivate the healthy X chromosome in individuals with Fragile X Syndrome. Jeannie Lee’s findings illustrate how unlocking these silenced X-linked genes could provide significant relief for those affected, thus transforming the landscape of treatment for this genetic condition.
| Key Points | Details |
|---|---|
| Understanding X-inactivation | Females have two X chromosomes but only one is active to equalize gene dosage with males. |
| Role of Xist | The gene Xist produces RNA that modifies the surrounding chromosomal material, facilitating the inactivation process. |
| Mechanism of Inactivation | Xist alters the properties of the ‘Jell-O’ substance surrounding chromosomes, making it more flexible and allowing other molecules to inactivate the X chromosome. |
| Potential Treatments | Lee’s lab is exploring methods to reactivate the inactivated X chromosome, which may offer treatments for conditions like Fragile X and Rett Syndrome. |
| Clinical Applications | Research suggests restoring the function of mutated genes may be achievable, minimizing side effects on healthy genes. |
| Future Directions | Continued research aims to refine therapies and transition to clinical trials over the coming years. |
Summary
X-inactivation mechanisms play a crucial role in gene dosage compensation between females and males, where one of the two X chromosomes in females is silenced. Recent discoveries by Jeannie Lee and her team reveal how the X chromosome’s surrounding material can be modified to facilitate this inactivation. Understanding these mechanisms opens the door to potentially novel treatments for genetic disorders linked to the X chromosome, such as Fragile X and Rett Syndromes. With ongoing advancements in this research, the hope for effective therapies becomes more tangible.